
myTXTL Sigma 70 Master Mix Kit
All-in-One Solution – Convenient reaction setup with Cell-Free Master Mix.
High Performance – Typically yields 1.5 mg/mL of protein (20 μg protein per reaction).
Fast Processing – Save time by avoiding transformation, clone selection and cell lysis.
- Product description
- Performance
- Publications
A rapid, easy-to-use cell-free master mix for protein expression in vitro, based on E. coli σ70 gene expression.
Cell-free expression platforms allow rapid and inexpensive protein synthesis with the highest versatility and flexibility due to their open-reaction character. This permits increased control over efficient substrate handling, and conveniently promotes precise online reaction monitoring and direct process optimization. A cell-free master mix removes the need for combining multiple components before expression to deliver robust performance and consistent results.
The myTXTL® technology is well-characterized and has been employed for various applications in protein engineering and synthetic biology applications. myTXTL Sigma 70 Master Mix is the perfect choice for high-yield production of soluble and membrane proteins, rapid prototyping of gene networks using plasmid DNA and RNA, the construction of synthetic minimal cells and to study cellular biology. Cell-free master mix on automation and miniaturization systems is ideal for library screening and delivers fast iteration of design-build-test cycles in protein and enzyme engineering.
Sigma 70 Cell-Free Master Mix Delivers:
All-in-One Solution – Convenient reaction setup with Cell-Free Master Mix.
High Performance – Typically yields 1.5 mg/mL of protein (20 μg protein per reaction).
Fast Processing – Save time by avoiding transformation, clone selection and cell lysis.
Highly Compatible – Accepts various constitutive and inducible E. coli and phage (T7, T3) promoters.
Various Inputs – Start from DNA or RNA templates.
Highly Controllable – Easily adjust experimental parameters
High-throughput Library Screening – Automation friendly for protein engineering studies.
Kit Specs
| Ordering Information | Size | Cat# |
| myTXTL Sigma 70 Master Mix Kit | 24 Reactions | 507024 |
| 96 Reactions | 507096 | |
| myTXTL Sigma 70 Master Mix – Bulk | 5 ml | 507005 |
| 25ml | 507025 | |
| myTXTL GamS Protein | 24 Reactions | 501024 |
| 96 Reactions | 501096 | |
| 384 Reactions | 501038 | |
| pTXTL-P70a-T7rnap HP | 24 Reactions | 502134 |
| 96 Reactions | 502135 | |
| 384 Reactions | 502139 | |
| pTXTL-P70a(2)-deGFP HP | 10 Reactions | 502138 |
| pTXTL-P70a-deGFP HP | 10 Reactions | 502117 |
| pTXTL-T7p14-deGFP HP | 10 Reactions | 502136 |
| E. coli KL740 cI857+ | —— | 502000 |
All-in-One Solution
myTXTL®Sigma 70 Master Mix is based on the TXTL technology developed by Prof. Vincent Noireaux at the University of Minnesota, and has been engineered for maximum robustness and reliable performance to meet our customer’s needs. The myTXTL®Sigma 70 Master Mix Kit contains E. coli cell extract, energy buffer and amino acids mix ready-to-use for in vitro protein production in a single tube, which is simply started by adding a nucleotide template. A positive control plasmid is included in the kit for simple confirmation of proper protein synthesis conditions when paired with the cell-free master mix.
As in vitro protein synthesis in the myTXTL®system relies on the endogenous core RNA polymerase and primary sigma factor 70 (σ70) of E. coli, a σ70-specific promoter is mandatory to express the gene of interest. For example, the lambda phage promoter encoded on our P70a vectors provides excellent protein yield for many proteins comparable to the T7 expression system. Gene expression with a T7 promoter can be easily accomplished with the myTXTL® T7 Expression Kit which provides continuous expression of T7 RNA polymerase.
Gene Circuits
For your convenience, Daicel Arbor Biosciences offers a large collection of over one hundred pre-designed plasmids for setting up complex gene circuits with the myTXTL® Toolbox 2.0 Plasmid Collection.
If a project occasionally requires linear DNA templates such as PCR products and myTXTL®Sigma 70 Master Mix in house, supplementing the Master Mix with GamS Purified Nuclease Inhibitor Protein is a perfect choice.
Custom & Bulk Formats
myTXTL®Sigma 70 Master Mix is now available in bulk formats for high-throughput processing and custom applications.
Fig 1. Protein Production in myTXTL Cell-Free Expression System (12-160 kDa)
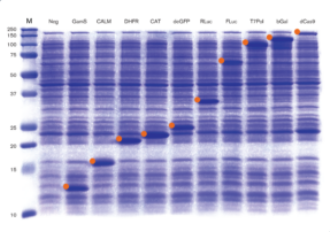
12 µL myTXTL reactions were set up with 5-10 nM template DNA (~ 30-650 ng DNA) carrying either a P70a or T7 promoter, and incubated for 16 hours at 29°C. After acetone precipitation, 1 µL of each reaction was loaded onto an SDS-PAGE gel and stained with Coomassie. Target proteins are marked with an orange identifier. M; Protein Marker (10-250 kDa); Neg; no template DNA; GamS; Truncated nuclease inhibitor protein Gam, CALM; Calmodulin-like protein 3, DHFR; Dihydrofolate reductase, CAT; Chloramphenicol acetyl transferase, deGFP; Variant of enhanced GFP, RLuc; Renilla luciferase, FLuc; Firefly Luciferase, T7Pol; T7 RNA polymerase, bGal; Beta-Galactosidase, dCas9; dead Cas9.
Fig 2. Production of positive control deGFP using myTXTL
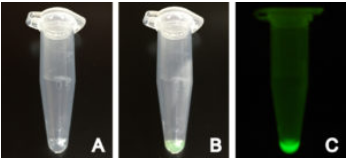
myTXTL Sigma 70 Master Mix with deGFP control plasmid before (A) and after (B) incubation at 29°C in a 1.5 mL reaction tube. (C) Fluorescence emitted by produced deGFP under UV light.
Fig 3. Effect of plasmid concentration on in vitro protein production.
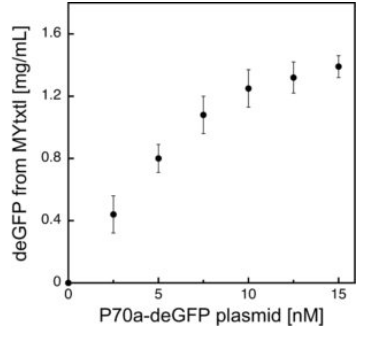
deGFP expression is regulated by the interaction of the endogenous E. coli core RNA polymerase and the primary sigma factor 70 (σ70) with the σ70-specific promoter P70a.
Fig 4. Protein synthesis in myTXTL using the T7 expression system.
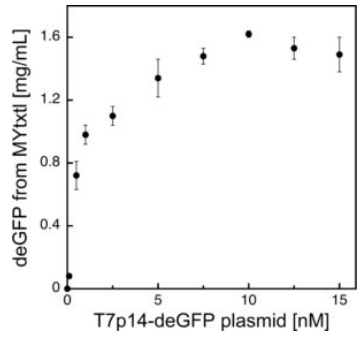
deGFP expression under the control of the bacteriophage T7 promoter (PT7) is facilitated by initial expression of T7 RNA polymerase from an additional plasmid.
Featured Publications
Liao, C. et al. (2018). The Francisella novicida Cas12a is sensitive to the structure downstream of the terminal repeat in CRISPR arrays. RNA Biol.
Marshall, R. et al. (2018). Rapid and scalable characterization of CRISPR technologies using an E. coli cell-free transcription-translation system. Molecular Cell.
Watters, K.E. et al. (2018). Systematic discovery of natural CRISPR-Cas12a inhibitors. Science.
Garamella J. et al. (2016). The all E. coli TX-TL toolbox 2.0: a platform for cell-free synthetic biology. ACS Synth Biol.
Caschera, F. et al. (2016). Compartmentalization of an all-E. coli Cell-Free Expression System for the Construction of a Minimal Cell. Artificial Life.
Takahashi, M. K. et al. (2015). Rapidly characterizing the fast dynamics of RNA genetic circuitry with cell-free transcription-translation (TX-TL) systems. ACS Synthetic Biology.








