
myTXTL T7 Expression Kit
All-in-one Solution – Simply mixing template DNA and ready-to-use myTXTL Master Mix.
High-throughput screening – Process more samples within a single experiment.
Various inputs – Start from linear or circular DNA templates.
- Product description
- Performance
Optimized for cell-free gene expression from T7-driven templates, either linear or circular DNA.
The myTXTL® T7 Expression kit is based on our LS70 Master Mix technology utilized in the myTXTL Linear DNA Expression Kit, and has been engineered for maximum robustness and reliable performance when using T7 promoter-driven constructs. This all-in-one solution contains a P70a-T7rnap HP plasmid which delivers continuous production of T7 RNA polymerase in addition to an E. coli-based master mix containing the cellular machinery, energy buffer, and amino acids premixed for in vitro protein production in a single tube. Simply start protein synthesis by adding a DNA template containing a T7 promoter. A positive control plasmid with one of our highest expressing green fluorescent proteins (GFP) is included in the kit for easy confirmation of proper protein synthesis conditions.
As in vitro protein synthesis in the myTXTL cell-free system relies on the endogenous primary sigma factor 70 (σ70) of E. coli, a σ70-specific promoter is used to drive expression of T7 RNA polymerase in the myTXTL T7 Expression kit. This co-expression of the P70a-T7rnap HP plasmid delivers continuous expression of T7 RNA polymerase for robust gene expression from traditional T7 expression system constructs.
All-in-one Solution – Simply mixing template DNA and ready-to-use myTXTL Master Mix.
High-throughput screening – Process more samples within a single experiment.
Various inputs – Start from linear or circular DNA templates.
Highly Controllable – Easily adjust experimental parameters.
Kit Specs
| Ordering Information | Size | Cat# |
| myTXTL T7 Expression Kit | 24 Reactions | 505024 |
| 96 Reactions | 505096 | |
| pTXTL-T7p14-deGFP HP | 10 Reactions | 502136 |
| pTXTL-P70a-T7rnap HP | 24 Reactions | 502134 |
| 96 Reactions | 502135 | |
| 384 Reactions | 502139 | |
| E. coli KL740 cI857+ | —— | 502000 |
Utilizing Existing T7 Constructs
Daicel Arbor Biosciences understands many organizations have large collections of genes already cloned into traditional T7 expression system constructs for classic E. coli expression. We created the myTXTL T7 Expression kit specifically for these individuals in order to deliver rapid analysis of gene expression from existing libraries. As the system can express numerous constructs simultaneously, the co-expression of P70a-T7rnap in no way hinders the expression of T7 promoter driven constructs and also allows for multiple T7-driven plasmids or linear DNA templates to be co-expressed in the system at once.
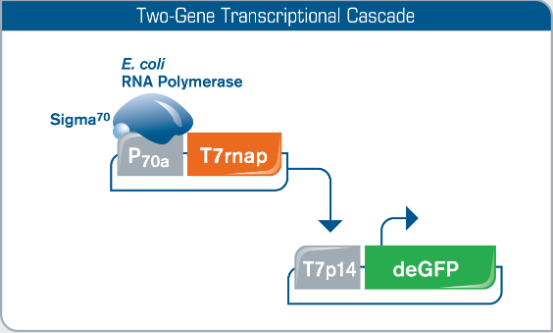
Fig 1. Continuous Expression of T7 RNA Polymerase. A T7 RNA Polymerase plasmid is co-expressed with a T7 promoter driven deGFP construct to generate a two-gene transcriptional cascade.
Gene Circuits
For your convenience, Daicel Arbor Biosciences offers a large collection of over one hundred pre-designed plasmids, including T7 promoter driven constructs, for setting up complex gene circuits with the myTXTL Toolbox 2.0 Plasmid Collection.
Fig 1. Protein Production in myTXTL Cell-Free Expression System (12-160 kDa)
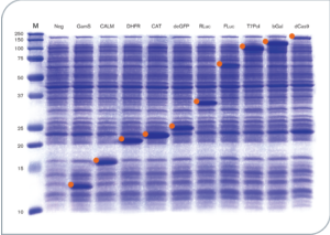
12 µL myTXTL reactions were set up with 5-10 nM template DNA (~ 30-650 ng DNA) carrying either a P70a or T7 promoter, and incubated for 16 hours at 29°C. After acetone precipitation, 1 µL of each reaction was loaded onto an SDS-PAGE gel and stained with Coomassie. Target proteins are marked with an orange identifier. M; Protein Marker (10-250 kDa); Neg; no template DNA; GamS; Truncated nuclease inhibitor protein Gam, CALM; Calmodulin-like protein 3, DHFR; Dihydrofolate reductase, CAT; Chloramphenicol acetyl transferase, deGFP; Variant of enhanced GFP, RLuc; Renilla luciferase, FLuc; Firefly Luciferase, T7Pol; T7 RNA polymerase, bGal; Beta-Galactosidase, dCas9; dead Cas9.
Fig 2. Protein synthesis in myTXTL using the T7 expression system.
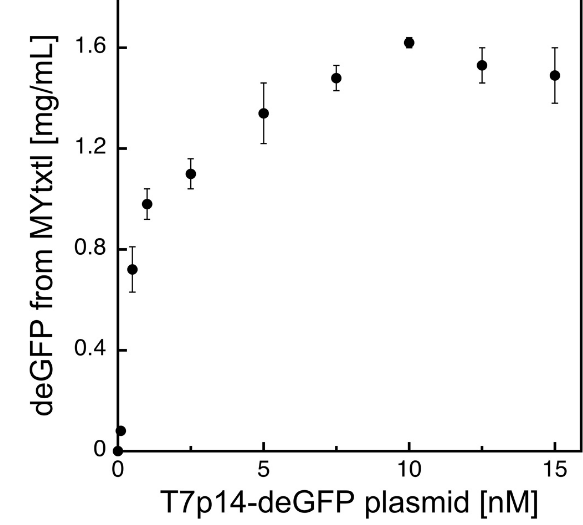
deGFP expression under the control of the bacteriophage T7 promoter (PT7) is facilitated by initial expression of T7 RNA polymerase from an additional plasmid.








