
myTXTL Linear DNA Expression Kit
All-in-one Solution – Simply mixing template DNA and ready-to-use myTXTL Master Mix.
Quick Turnaround – Gene expression directly from PCR product
High-throughput screening – Process more samples within a single experiment.
- Product description
- Performance
- Publications
Optimized for cell-free protein synthesis from linear DNA template molecules.
The myTXTL® Linear DNA Expression Kit is based on Daicel Arbor Biosciences’ popular myTXTL® Sigma 70 Master Mix Kit, which has been further engineered to efficiently produce soluble and membrane proteins using linear DNA templates without the need for additional stabilizers. Simply add linear DNA template to the optimized master mix to begin protein synthesis.
This kit is particularly useful for the screening of DNA libraries that were generated by PCR amplification. In combination with the ability to utilize an automated liquid handling system for master mix dispensing, myTXTL Linear DNA Expression Kit is ideal for high-throughput applications. The myTXTL Linear DNA Expression Kit is also recommended to produce live bacteriophages starting from their whole RNA or DNA genome. This can be employed to study phage therapy, a promising approach to treat pathogenic bacterial infections.
All-in-one Solution – Simply mixing template DNA and ready-to-use myTXTL Master Mix.
Quick Turnaround – Gene expression directly from PCR product
High-throughput screening – Process more samples within a single experiment.
Various expression systems – Compatible with E. coli-specific and T7 expression systems.
Kit Specs
| Ordering Information | Size | Cat# |
| myTXTL Linear DNA Expression Kit | 24 Reactions | 508024 |
| 96 Reactions | 508096 | |
| myTXTL Linear DNA Master Mix – Bulk | 5 ml | 508005 |
| 25 ml | 508025 | |
| P70a-deGFP Positive Control Fragment | 5 Reactions | 508009 |
| pTXTL-P70a-T7rnap HP | 24 Reactions | 502134 |
| 96 Reactions | 502135 | |
| 384 Reactions | 502139 | |
| E. coli KL740 cI857+ | —— | 502000 |
The myTXTL Linear DNA Expression Kit contains an engineered E. coli cell extract, energy buffer and amino acids mix ready-to-use for in vitro protein production in a single tube. In vitro protein synthesis is initialized by adding a linear DNA fragment. A linear fragment of P70a-deGFP plasmid is included in the kit as the positive control. Additionally, myTXTL Linear DNA Expression master mix is available in bulk formats for high-throughput processing and custom applications.
As in vitro protein synthesis in the myTXTL system relies on the endogenous core RNA polymerase and primary sigma factor 70 (σ70) of E. coli, a σ70-specific promoter is mandatory to express the gene of interest. For example, the lambda phage promoter encoded on our P70a vectors provides excellent protein yield for many proteins comparable to the T7 expression system.
Fig 1. Protein Production in myTXTL Cell-Free Expression System (12-160 kDa)
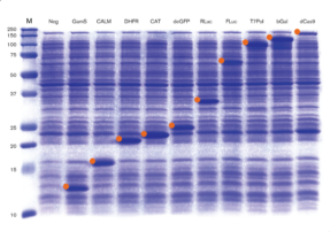
12 µL myTXTL reactions were set up with 5-10 nM template DNA (~ 30-650 ng DNA) carrying either a P70a or T7 promoter, and incubated for 16 hours at 29°C. After acetone precipitation, 1 µL of each reaction was loaded onto an SDS-PAGE gel and stained with Coomassie. Target proteins are marked with an orange identifier. M; Protein Marker (10-250 kDa); Neg; no template DNA; GamS; Truncated nuclease inhibitor protein Gam, CALM; Calmodulin-like protein 3, DHFR; Dihydrofolate reductase, CAT; Chloramphenicol acetyl transferase, deGFP; Variant of enhanced GFP, RLuc; Renilla luciferase, FLuc; Firefly Luciferase, T7Pol; T7 RNA polymerase, bGal; Beta-Galactosidase, dCas9; dead Cas9.
Fig 2. Production of positive control deGFP using myTXTL
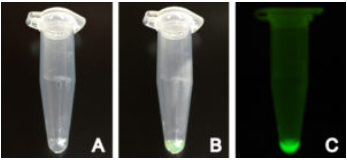
myTXTL Sigma 70 Master Mix with deGFP control plasmid before (A) and after (B) incubation at 29°C in a 1.5 mL reaction tube. (C) Fluorescence emitted by produced deGFP under UV light.
Fig 3. Effect of plasmid concentration on in vitro protein production.
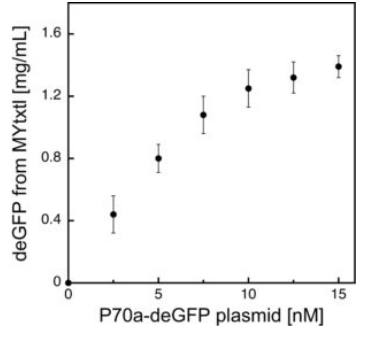
deGFP expression is regulated by the interaction of the endogenous E. coli core RNA polymerase and the primary sigma factor 70 (σ70) with the σ70-specific promoter P70a.
Fig 4. Protein synthesis in myTXTL using the T7 expression system.
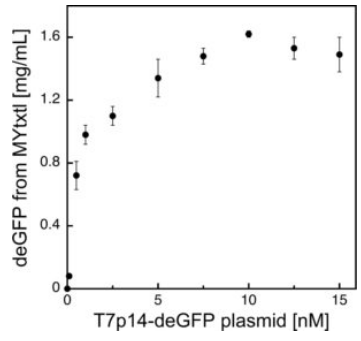
deGFP expression under the control of the bacteriophage T7 promoter (PT7) is facilitated by initial expression of T7 RNA polymerase from an additional plasmid.
Featured Publications
Rustad M. et al. (2018). Cell-free TXTL synthesis of infectious bacteriophage T4 in a single test tube reaction. Synthetic Biology.
Marshall, R. et al. (2017). Short DNA containing χ sites enhances DNA stability and gene expression in E. coli cell-free transcription-translation systems. Biotechnology and Bioengineering.
Garamella J. et al. (2016). The all E. coli TX-TL toolbox 2.0: a platform for cell-free synthetic biology. ACS Synth Biol.
Shin, J. et al. (2012). Genome replication, synthesis, and assembly of the bacteriophage T7 in a single cell-free reaction. ACS Synthetic Biology.








