
myTXTL Toolbox 2.0 Plasmid Collection
This is an excellent source for designing comprehensive gene circuits.
It also an ideal for educational purposes to train students and demonstrate basic concepts of synthetic biology.
Environmental sensors, controls for biomanufacturing of biofuels, stem cell medicine, gene therapy (CRISPR), and manufacturing of functional materials.
- Product description
- Performance
- Publications
The myTXTL Toolbox 2.0 Plasmid Collection is an excellent source for designing comprehensive gene circuits.
The combination of synthetic gene networks with in vitro protein production technology opens new innovative fields of application in medicine and biotechnology. Examples are environmental sensors, controls for biomanufacturing of biofuels, stem cell medicine, gene therapy (CRISPR), and manufacturing of functional materials. Cell-free platforms help to overcome limitations occurring in living host organisms, which are typically tied to the biochemical cross-talk between the host cell and the artificial gene circuit and a consequence of the potentially cytotoxic effect of the exogeneous gene network on the living cell on the one hand, and the availability of resources for transcription and translation, DNA replication and metabolites on the other. As those resources are dependent on the cell density, growth rate and cultivation conditions, deploying cell-free systems for gene circuit testing creates a more controlled environment thus increasing reproducibility and robustness of the circuit behavior and output.
Kit Specs
| Ordering Information | Cat# |
| pTXTL-P19a-S24 | 502005 |
| pTXTL-P19a-S28 | 502007 |
| pTXTL-P19a-S28-ssrA | 502008 |
| pTXTL-P19a-S38 | 502009 |
| pTXTL-P19a-deCFP | 502002 |
| pTXTL-P19a-deGFP | 502001 |
| pTXTL-P19a-deYFP | 502003 |
| pTXTL-P19a-ntrC | 502004 |
| pTXTL-P19a-ompA-S24 | 502006 |
| pTXTL-P24a-S28 | 502011 |
| pTXTL-P24a-S28-ssrA | 502013 |
| pTXTL-P24a-S38 | 502014 |
| pTXTL-P24a-deGFP | 502010 |
| pTXTL-P24a-ompA-S38 | 502012 |
| pTXTL-P28a-aH | 502015 |
| pTXTL-P28a-aH-eGFP | 502018 |
| pTXTL-P28a-cI-ssrA | 502017 |
| pTXTL-P28a-S19 | 502031 |
| pTXTL-P28a-S19-ssA | 502032 |
| pTXTL-P28a-S24-ssrA | 502033 |
| pTXTL-P28a-S38 | 502035 |
| pTXTL-P28a-S54 | 502036 |
| pTXTL-P28a-T7rnap | 502118 |
| pTXTL-P28a-deGFP | 502019 |
| pTXTL-P28a-deGFP-ssrA | 502020 |
| pTXTL-P28a-deYFP | 502021 |
| pTXTL-P28a-flgM | 502022 |
| pTXTL-P28a-flgM-ssrA | 502023 |
| pTXTL-P28a-mApple | 502025 |
Gene Circuits
The myTXTL Toolbox 2.0 Plasmid Collection was designed and intensively studied by Vincent Noireaux, PhD at the University of Minnesota and co-workers. It contains over one hundred plasmids with various promoters and open reading frames (ORFs) to investigate gene regulation and molecule turnover. Available open reading frames include a wide selection of transcription factors, TXTL modulators and fluorescent reporter proteins to build multi-stage gene circuits.
A gene circuit executed in any myTXTL® kit is required to start with a σ70-specific promoter, as gene expression in myTXTL® relies entirely on the endogenous TXTL machinery of E. coli. Its modular and pre-designed format makes it also ideal for educational purposes to train students and demonstrate basic concepts of synthetic biology.
The myTXTL Toolbox 2.0 Plasmid Collection serves as a comprehensive source to build a variety of gene circuits executable in the myTXTL system. The examples below highlight selected features and capabilities. For more inspiration, visit our publication page and choose the keyword “gene circuit”.
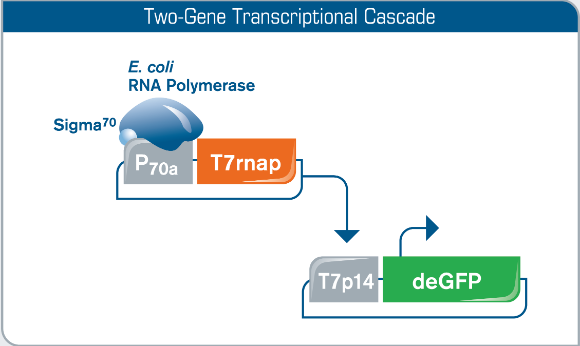
Fig 1. Utilizing a Two-Gene Transcriptional Cascade for T7 Promoter Expression. To facilitate T7 promoter regulated gene expression in myTXTL, T7 RNA polymerase is co-expressed from a helper plasmid (P70a-T7rnap) encoding a Sigma70-specific promoter compatible with myTXTL Master Mixes. T7 RNA polymerase then facilitates gene expression on the T7 promoter DNA template (here: T7p14-deGFP plasmid). Constitutive and inducible T7 promoter versions are acceptable.
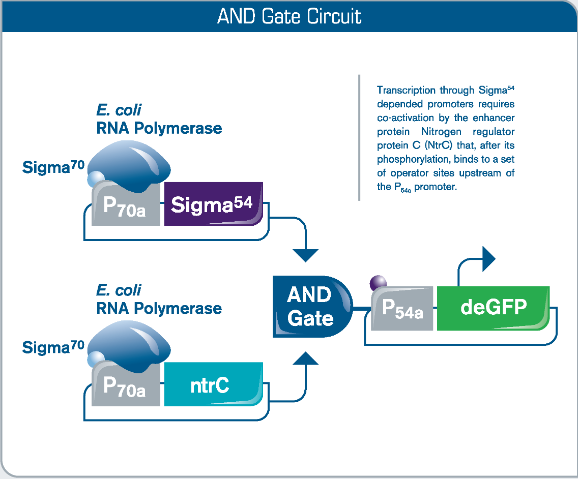
Fig 2. Execution of Biologic Logic Gates in myTXTL. In synthetic biology, logic gates research is aimed at the development of decision-making gene networks for environmental and medical applications, in which biological gates act as sensors, detection units and drug vehicles. In this AND gate circuit, deGFP expression requires both phosphorylation of Nitrogen regulator protein C (NtrC) as well as presence of transcription factor Sigma54.
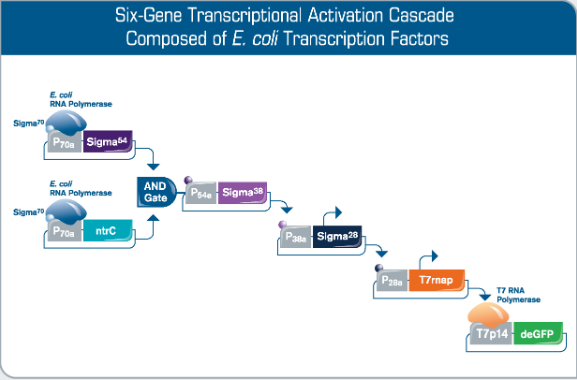
Fig 3. Rapid Prototyping of Synthetic Gene Networks. Engineering of synthetic gene networks involves executing an iterative design-build-test cycle which is accelerated by cell-free expression technology like myTXTL. By utilizing the native E. coli TXTL machinery, myTXTL gives access to an extraordinarily large repertoire of genetic building blocks. Here, a multi-stage cascade composed of a logic AND gate and bacterial transcription factors is producing a fluorescent output. Other fluorescent proteins are part of the myTXTL Toolbox 2.0 Plasmid Collection.
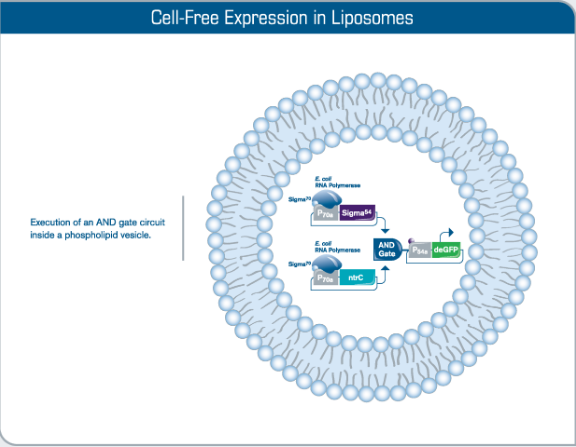
Fig 4. Compartmentalization of Cell-Free Reactions. The encapsulation of myTXTL reactions into large phospholipid vesicles (liposomes) is an example of how artificial cells hosting cellular functions and complexity as advanced as in nature are constructed. Incorporation of transmembrane proteins provides more sensing options and facilitates cellular communication. Other approaches of compartmentalization such as emulsions, liquid-liquid phase separation, hydrogels and polymer-containing membrane compartments can also be applied.
Featured Publications
Yelleswarapu M. et al. (2018). Sigma factor-mediated tuning of bacterial cell-free synthetic genetic oscillators.ACS Synth Biol.
Garamella J. et al. (2016). The all E. coli TX-TL toolbox 2.0: a platform for cell-free synthetic biology.ACS Synth Biol.








